Metal soaps
A common and widespread issue in oil paint conservation is the formation of so-called ‘metal soaps’: complexes of metal ions (usually lead or zinc) with long-chain fatty acids. These complexes are the result of reactions between pigments or driers with the oil binder. The problem occurs especially when the ester bonds in the oil molecules that make up the polymerised oil network are hydrolysed to some degree. The presence of metal soap phases has been linked to many types of paint deterioration, such as brittleness, delamination and transparency of paint layers, as well as the formation of disfiguring protrusions on paint surfaces.
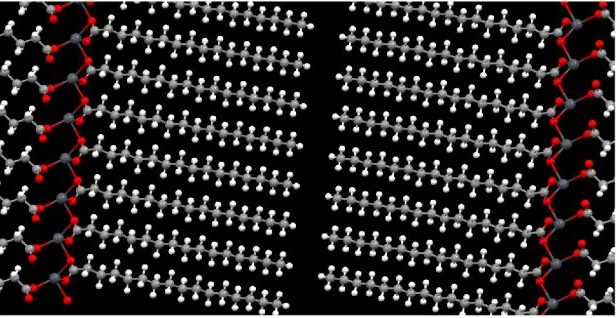
In my PhD research, it was my aim to unravel the pathway of chemical reactions that lead to metal soap formation in oil paintings. To reach this goal, the methods to resolve the structure of various kinds of crystalline lead and zinc soaps were improved with a combination of IR spectroscopy and powder-XRD. The solubility and crystallisation kinetics of metal soaps were investigated with DSC, leading to phase diagrams of metal soaps in linseed oil media (1). By creating an ‘ionomer’ model systems that mimic the structure of aged oil paint, it was then possible to prove that metal ions from pigments are released into the oil binder to form ionic metal-carboxylate clusters (2). This result greatly helped the interpretation of IR spectra of samples from real paintings, and provided a basis for detailed kinetic studies on metal soap formation. For instance, we used IR spectroscopy to measure the diffusion of fatty acids and their reaction kinetics with metal ions leading to metal soap formation in oil paint binders (3).

